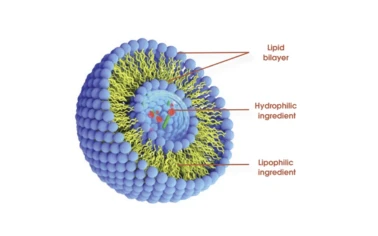
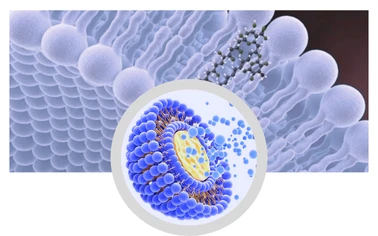
acerola cherry powder suppliers
Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API is a key solution in the Pharmaceutical manufacturing industry, specifically within Manufacturing of raw materials for chemical drugs and Plant derived chemical raw materials. This article explores how Hebei Finutra Biotech Co., Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors. Table of Contents Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API Overview Benefits & Use Cases of Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API in Plant derived chemical raw materials Cost, Maintenance & User Experience Sustainability & Market Trends in Pharmaceutical manufacturing Conclusion on Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API from Hebei Finutra Biotech Co., Ltd. Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API Overview As flavonoid glycosides derived from citrus peels, Diosmin and Hesperidin are widely adopted as plant-based pharmaceutical raw materials and APIs across regulated and emerging markets. In manufacturing, they serve in vascular-support formulations and as starting materials for further derivatization (e.g., aglycone conversions). For B2B teams, the key drivers are consistency, regulatory readiness, and robust technical documentation. Hebei Finutra Biotech Co., Ltd. supplies Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API with process control designed for reproducibility from pilot to commercial scale. Technically, materials are offered in standard and micronized grades to support compressibility, blend uniformity, and dissolution targets. Typical controls include HPLC assay and related substances, ID by UV/IR, microbial quality, residual solvents aligned with ICH Q3C, heavy metals per ICH Q3D, and pesticide residues suited to plant-derived APIs. Packaging options (commonly 25 kg fiber drums with double PE liners) protect against moisture and light, with stability data available to support shelf life and retest periods. Finutra’s quality system emphasizes GMP-oriented production, ISO-driven management, and method validation, helping sponsors accelerate qualification while mitigating audit risk. Anonymized customer feedback highlights reduced batch deviations and smoother scale transfers attributable to consistent particle size distribution and clean impurity profiles. Benefits & Use Cases of Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API in Plant derived chemical raw materials In the plant-derived chemicals category, Diosmin and Hesperidin offer a reliable path to natural-origin APIs and intermediates. Use cases include direct API deployment in oral solid dosage forms, premix granulation for improved flow, and as intermediates for downstream flavonoid transformations. Micronized Diosmin can enhance blend homogeneity in high-load tablets, while standard Hesperidin serves as a versatile building block for value-added derivatives. For nutraceutical-adjacent applications, quality parameters align with pharmaceutical expectations, streamlining dual-channel commercialization where allowed by local regulation. Key advantages from Hebei Finutra Biotech Co., Ltd. include tight control of botanical sourcing, scalable extraction and purification, and customizable specs (assay ranges, particle size targets, color/appearance requirements). The company’s process routes emphasize solvent recovery and low-impurity crystallization, which supports consistent dissolution profiles and compressibility. Finutra’s cross-functional team—process, QA/QC, and regulatory—provides rapid documentation support (e.g., comprehensive COAs, method validation packages, stability summaries) that facilitates smoother technical transfers and supplier qualifications for global pharma and CDMO partners. In short, Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API from Finutra helps manufacturers balance performance, compliance, and sourcing resilience. Cost, Maintenance & User Experience Total cost of ownership in chemical drug manufacturing is driven by more than price per kilogram. With Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API, B2B buyers often realize savings through reduced in-house milling (via micronized grades), fewer OOS events (consistent impurities and PSD), and streamlined QA thanks to complete, audit-ready documentation. Finutra’s multi-origin citrus peel supply and controlled extraction scheduling help stabilize availability and pricing, while buffer stocks and flexible MOQs can shorten lead times and mitigate stockout risk. From a “maintenance” perspective, these APIs are low-touch when stored correctly: keep sealed in original packaging, protected from light, at cool, dry conditions with standard GDP handling. Users report dependable flow in granulation, predictable compression behavior, and clean lubrication profiles—factors that reduce rework and protect cycle times. Customers in the Manufacturing of raw materials for chemical drugs sector value Finutra’s responsive technical support, transparent change control, and lot-to-lot reproducibility, which collectively support higher asset utilization and stronger ROI across the product lifecycle. Sustainability & Market Trends in Pharmaceutical manufacturing Plant-derived APIs continue to gain traction as pharma sponsors pursue diversification, resilience, and greener chemistry. Regulatory expectations emphasize traceability from botanical source through finished API, risk-based contaminant control, and alignment with ICH Q7/Q11, pharmacopeial monographs, and EHS standards. Market growth is supported by circular-economy thinking—valorizing citrus peel byproducts from the juice industry—alongside solvent minimization, recovery, and energy-efficient processing. These trends favor suppliers who can document end-to-end stewardship and data integrity across the supply chain. Hebei Finutra Biotech Co., Ltd. positions itself at this intersection of compliance and sustainability. The company prioritizes responsible sourcing, ethanol/water-centric extraction where applicable, solvent recovery systems, and waste reduction initiatives. Continuous improvement frameworks, quality-by-design principles, and environmental management practices underscore Finutra’s commitment to eco-conscious manufacturing without compromising technical performance. For buyers, this translates into a dependable, future-ready supply of Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API with the documentation and transparency needed to satisfy internal governance and external audits. Conclusion on Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API from Hebei Finutra Biotech Co., Ltd. For decision-makers in Pharmaceutical manufacturing—especially within the Manufacturing of raw materials for chemical drugs and Plant derived chemical raw materials—Diosmin Citrus Aurantium Extract Hesperidin Pharmaceutical Chemicals API delivers a compelling mix of quality, consistency, and sustainability. Hebei Finutra Biotech Co., Ltd. couples robust process control with strong technical and regulatory support, enabling efficient onboarding and dependable long-term supply. Whether you seek standard or micronized grades, tight impurity profiles, or tailored documentation, Finutra offers a partnership model built for scale and compliance. Contact us: info@finutra.com Visit our website: https://www.finutra.com
Finutra devotes to be an integrated supplier for global supply chain, we offer a
broad array of raw materials and functional ingredients
Authoritative Certification
Continuous Innovation, Customer First
Enhance core competitiveness to bring customers better products and services,
Each of these is the result of our team's relentless pursuit of excellence
and our deep commitment to social responsibility.








Global
Reach
FINUTRA has over 350,000 square feet of manufacturing and warehouse
space worldwide.
Industries We Serve
Advanced molecular distillation and microencapsulation
technology. Extremely bioavailable
trace carotenoids Intuitively soluble.


STAY UPDATED
Receive special offers and first look at new
products.
products.
Building 23B1, No.2 Yuanboyuan St., Zhengding Area of China (Hebei) Pilot Free Trade Zone
QUICK LINK
Finutra devotes to be an integrated supplier for global supply chain, we offer a broad array of raw
materials and functional ingredients as a manufacturer, distributor and supplier for global Beverage,
Nutraceutical, Food, Feed and Cosmeceutical.
Copyright © 2025 Hebei Finutra
Biotech Co.,
Ltd. All
Rights Reserved.
Privacy Policy